Magnesium and Copper Ii Sulfate Net Ionic Equation
Magnesiumcopper2 sulfate-- magnesium sulfate copper. Does a reaction occur when aqueous solutions of magnesium nitrate and cobaltII chloride are combined.

How To Write The Net Ionic Equation For Mg Cuso4 Cu Mgso4 Youtube
There will be no net reaction between MgSO4 and Ni View the full answer Transcribed image text.
. 2 H2aq CO 32-aq ---- CO g H 2 Ol charge. Iron is oxidised and copper is reduced. 2 -2 0 0 0 a Overall equation.
Fe s Cu 2aq Fe 2aq Cu s This is the ionic equation for the reaction between iron and copper II sulfate. Magnesium becomes coated with copper the blue colour of the. Mg CuSO4 MgSO4 Cu Theoretically if Magnesium is placed in Copper Sulfate solution a single replacement reaction occurs.
What is the ionic equation for the reaction between magnesium and copper II sulfate. For the reaction between magnesium and copper II sulfate solution is. Cu² aq Zn s Cus Zn²aq Write a net ionic equation for the reaction of copperII carbonate and hydrochloric acid.
Magnesium copper sulfate balanced equation. Mg Cu2 -----. Mg CuSO4 MgSO4 Cu Magnesium Copper Sulfate Magnesium Sulfate Copper What is the chemical equation for nickel sulfate plus.
Similarly the reaction between tin and lead chloride may be written as Sn s Pb 2aq Sn 2aq. Write a net ionic equation for the reaction of copperII sulfate solution and zinc metal. Aq Cus Mg aq.
S 2-aq Pb 2 aq à PbSs 4. A balanced chemical equation. Write the ionic equation for the displacement reaction by adding the half equation.
Copper solid is displaced and the magnesium loses electrons to copper ions. How to determine the reactivity of magnesium sulfate. Mg sCu2aq Mg2aq Cu s The net ionic equation is different from the ionic equation because it does not contain the hydrated sulfate ions.
When two solutions of ionic compounds are mixed a solid may form. For example when magnesium Mg reacts with copper II sulfate CuSO4 the magnesium atoms react with the copper II ions in the copper II. 2 Na-aq SO 3 2aq 2 Haq 2 Cl-aq ---- spectato r spectato r.
The single replacement reaction of Magnesium and Copper Sulfate produce. Does a reaction occur when aqueous solutions of calcium nitrate and copperII sulfate are combined. Zn s Cu 2 aq Cu s Zn 2 aq Since the copper II ion has substantially greater reduction potential 015 V than zinc ion -076 V it is readily reduced by zinc metal.
Question 18 2 points The net ionic equation of the redox reaction of magnesium and copper II sulfate. Mg sCu2aq SO2- 4 aq Mg2aq SO2- 4 aq Cu s This gives the net ionic equation. Cu Mg2 Molecular equation of copper II sulfate sodium carbonate Net ionic equation.
Mg s CuSO 4 aq MgSO 4 aq. Identify the oxidizing and reducing agent from the below reaction oxidation and reduction half Mg s Cu. Magnesium Mg Copper sulphate CuSO4MolecularMgs CuSO4aq -- MgSO4aq CusComplete ionicMgs Cu2aq SO42-aq -- Mg2aq SO42-aq CusNet ionic equationMgs Cu2aq -- Mg2aq Cus answer.
Answer 1 of 4. Magnesium copper sulfate balanced equation magnesium copper sulfate balanced equation magnesium copper sulfate balanced equation gospel for sunday march 20 2022. This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules such as those found in the.
Phd viva voce examination report sample. You didnt specify whether it was copper I chloride or copper II chloride but either way all nitrates are soluble so the aqueous anion present would be NO3- with a. A balanced equation for the reaction between magnesium and copper sulfate solution can be written in terms of the ions involved.
What is the equation for copper sulfate and magnesium. CopperII sulfate barium chloride. Copper solid is displaced and the magnesium loses electrons to copper ions.
Keto whole trout recipe. Complete Ionic Equation Net Ionic Equation 10 Silver nitrate and magnesium from SLE 214 at Deakin University. Answer 1 of 8.
Theoretically if Magnesium is placed in Copper Sulfate solution a single replacement reaction occurs. 4 Select the net ionic equation for the reaction that occurs when magnesium sulfate and nickelII nitrate are mixed. Net ionic equation.
When two solutions of ionic compounds are mixed a solid may form. H 23 SO aq unstable substance Na 23 SO aq 2 HClaq 6 H 2 Ol SO 2 g 2 NaClaq soluble strong soluble salt acid salt b Total ionic equation. The single replacement reaction of Magnesium and Copper Sulfate produce.
The blue color of the aqueous copper II sulfate solution is due to the presence of the hexaaquacopper II ion in water. Honors Chemistry Name_____ Period_____ Net Ionic Equation Worksheet READ THIS. Ba 2 aq SO 4 2-aq à BaSO 4 s 5.
Mg CuSO4 MgSO4 Cu. If a reaction does occur write the net ionic equation. BaCl 2 aq CuSO 4 aq à BaSO 4 s CuCl 2 aq Net ionic.
Mg s Cu2aq SO42-aq Mg2aq SO42-aq Cu s How to write a balanced chemical equation for magnesium. C Net ionic equation. If a reaction does occur write the net ionic equation.
Magnesium copper II sulfate magnesium sulfate copper Mg s CuSO4aq MgSO4aq Cu s In this displacement reaction.
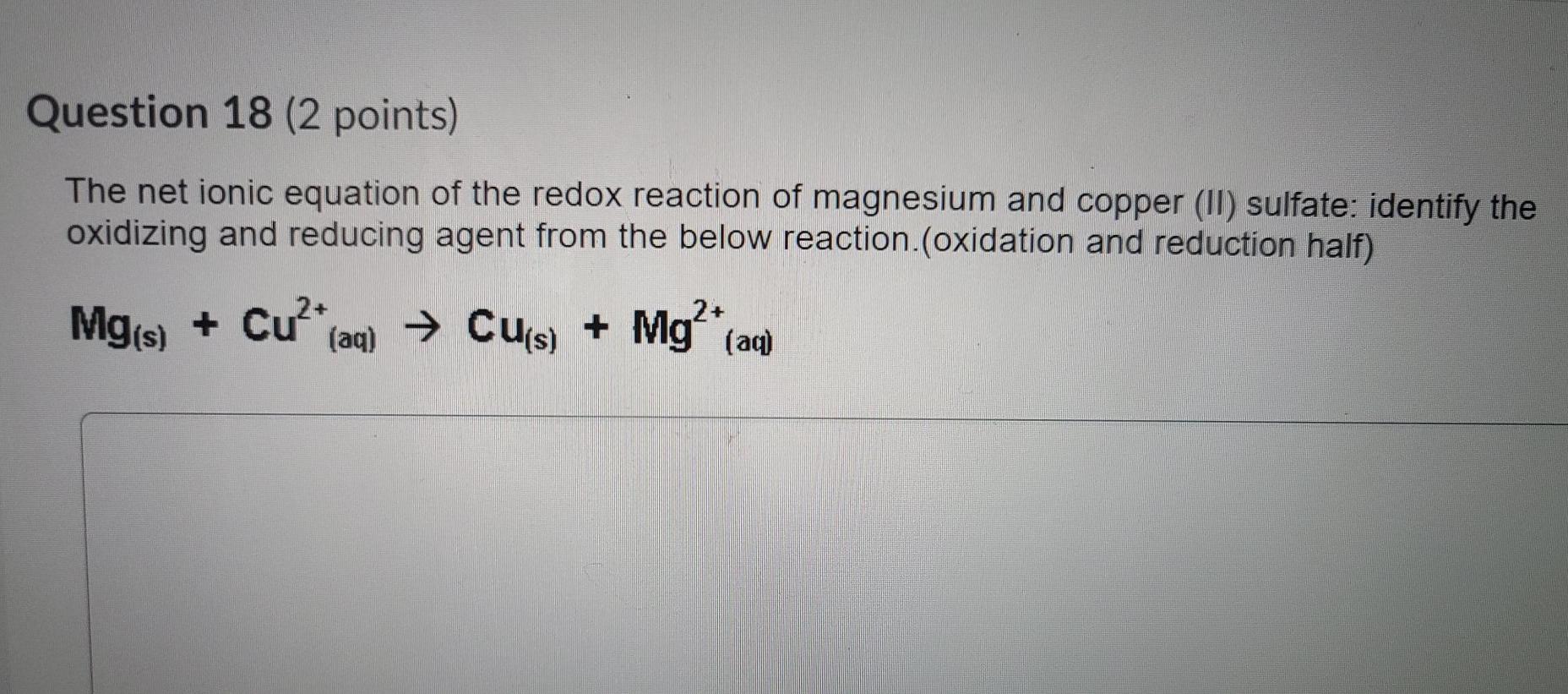
Solved Question 18 2 Points The Net Ionic Equation Of The Chegg Com

How To Write The Net Ionic Equation For Mg Cuso4 Cu Mgso4 Youtube

How To Write The Net Ionic Equation For Mg Cuso4 Cu Mgso4 Youtube
No comments for "Magnesium and Copper Ii Sulfate Net Ionic Equation"
Post a Comment